Targeted radionuclide diagnosis and therapy is an emerging field and has been already implemented in effective cancer treatment. The tumor-targeting moieties are generally designed to bind to cell surface receptors or biomolecules that are (over)expressed in the tumor microenvironment and have no or sufficiently low expression in non-tumor tissue. The tumor-seeking ability of targeted radionuclide therapies makes these therapies effective in the elimination of primary tumors, including any (undetected) metastasis. In addition, targeting may reduce side effects of the treatment as a result of the low exposure of healthy tissues. The targeting moiety of radiopharmaceuticals can precisely locate and guide radionuclides to the target, where radionuclides kill surrounding tumor cells. The development of new theragnostic radiopharmaceuticals, which can be used for both imaging and therapy purposes, allows for more precise targeting and monitoring of the treatment response. Effective application of radiopharmaceuticals depends on the selection of an appropriate targeting moiety and the right design of the complete molecule.
The most employed targeting moieties today include antibodies, peptides, nucleic acids, small molecules, and nanoparticles, each of which has advantages and disadvantages.
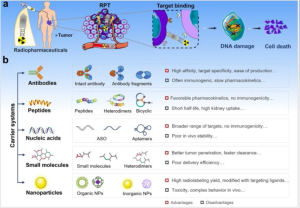
Zhang, T., Lei, H., Chen, X. et al. Carrier systems of radiopharmaceuticals and the application in cancer therapy. Cell Death Discov. 10, 16 (2024)
Antibodies were the first biological carriers employed for radiopharmaceuticals. The high affinity and specificity of antibodies for target antigens overexpressed on tumors make them excellent carriers for radionuclide delivery, especially monoclonal antibodies (mAbs) and their derivatives. However, intact mAbs have inherent main drawbacks due to their relatively high molecular weight, including slow pharmacokinetics and low diffusivity within solid tumors. Although antibody fragments can improve the pharmacokinetics of solid tumor therapies, they are reduced in stability and show a significant degree of nonspecific accumulation in healthy tissues.
In recent years, nucleic acids are also being explored as carriers of radiopharmaceuticals, which have some significant advantages in terms of production, modification, possible targets, and immunogenicity. Small molecule radiopharmaceuticals can easily penetrate tumors and are rapidly cleared from non-target tissues, thus reducing toxicity, compared to large molecules. Furthermore, advanced hybrid radionuclide carriers such as antibody- and peptide-modified nanoparticles have been studied.
There are some requirements which should be fulfilled in order to develop successful radiotracers. In general, radiotracers should be small in size, easy to synthesize and radiolabel. They should show high binding affinity for their receptors, high tumor uptake and retention as well as rapid clearance from the blood and non-target tissue. Additionally, they should be amenable to chemical and molecular modifications and a chelator should be attachable without affinity loss. Further adaptions like modification of linker length, selection of the most suitable chelator, overall charge of the molecule, introducing plasma protein binding entities, etc. might be helpful for the design of a good radiopharmaceutical.
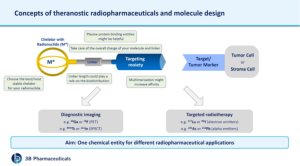
Radiolabeled peptides are relatively easily modifiable according to these requirements and are therefore valuable biological tools for tumor receptor imaging and targeted radionuclide therapy. Peptide-based radiopharmaceuticals were introduced into the clinic more than two decades ago. The first registered imaging agent was the somatostatin (SST) analog, 111In-DTPA0-octreotide (111In-OctreoScan, 111In-pentetreotide). Peptides have many advantages that make them attractive radiopharmaceutical carriers, including ease of synthesis and radiolabeling, favorable pharmacokinetics, low toxicity and immunogenicity. An issue related to radiolabeled peptides is often their high uptake and retention by the kidneys, which is a concern, particularly for radionuclide therapy because of the potential nephrotoxicity. The exact biological mechanisms underlying the accumulation of radioactivity in the kidneys are not fully identified, as evidence supports the involvement of multiple mechanisms for different radioligands. As amino acids are highly valuable to the body, cells in the renal tubules have developed mechanisms enabling the reabsorption of (small) proteins and peptides so that the body can reuse them. The right molecule design can help to significantly reduce this issue.
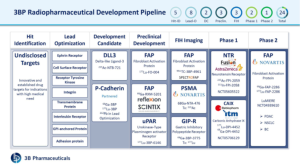
3B Pharmaceuticals GmbH (3BP), a German biotechnology company developing targeted radiopharmaceutical drugs and diagnostics for oncology indications with a high unmet medical need has therefore chosen peptides as their favourite molecules when it comes to radiotracers. As a leader in peptide discovery and optimization, 3BP has built a technology platform extending from hit identification to early clinical development.
Several programs like FAP- and CAIX-targeting peptides are being clinically developed by our partners Novartis and Debiopharm/itm, respectively. In addition to that, 3BP has a full pipeline of theranostic radiopharmaceuticals for various targets at different stages of development. 3BP is currently building and expanding its clinical development capabilities to translate its pipeline programs into early phase clinical trials.
References
Fani M, Maecke HR, Okarvi SM. Radiolabeled peptides: valuable tools for the detection and treatment of cancer. Theranostics. 2012;2(5):481-501. https://doi: 10.7150/thno.4024
Zhang, T., Lei, H., Chen, X. et al. Carrier systems of radiopharmaceuticals and the application in cancer therapy. Cell Death Discov. 10, 16 (2024), https://doi.org/10.1038/s41420-023-01778-3
de Roode KE, Joosten L, Behe M. Towards the Magic Radioactive Bullet: Improving Targeted Radionuclide Therapy by Reducing the Renal Retention of Radioligands. Pharmaceuticals (Basel). 2024 Feb 16;17(2):256. https://doi: 10.3390/ph17020256
Chakraborty, K.; Mondal, J.;An, J.M.; Park, J.; Lee, Y.-K. Advances in Radionuclides and Radiolabelled Peptides for Cancer Therapeutics. Pharmaceutics 2023, 15, 971. https://doi.org/10.3390/pharmaceutics15030971
Zboralski D, Hoehne A, Bredenbeck A, Schumann A. et al. Preclinical evaluation of FAP-2286 for fibroblast activation protein targeted radionuclide imaging and therapy. Eur J Nucl Med Mol Imaging. 2022 Sep;49(11):3651-3667. https://doi: 10.1007/s00259-022-05842-5
Baum RP, Schuchardt C, Singh A, Chantadisai M. et al. Feasibility, Biodistribution, and Preliminary Dosimetry in Peptide-Targeted Radionuclide Therapy of Diverse Adenocarcinomas Using 177Lu-FAP-2286: First-in-Humans Results. J Nucl Med. 2022 Mar;63(3):415-423. https://doi: 10.2967/jnumed.120.259192
Greifenstein L, Gunkel A, Hoehne A, Osterkamp F. et al. 3BP-3940, a highly potent FAP-targeting peptide for theranostics – production, validation and first in human experience with Ga-68 and Lu-177. iScience. 2023 Nov 23;26(12):108541. https://doi:10.1016/j.isci.2023.108541
Massière F, Wiedemann N, Borrego I, Hoehne A, et al. Preclinical Characterization of DPI-4452: A 68Ga/177Lu Theranostic Ligand for Carbonic Anhydrase IX. J Nucl Med. 2024 May 1;65(5):761-767. https://doi: 10.2967/jnumed.123.266309
Hofman MS, Tran B, Feldman DR, Pokorska-Bocci A, et al. First-in-Human Safety, Imaging, and Dosimetry of a Carbonic Anhydrase IX-Targeting Peptide, [68Ga]Ga-DPI-4452, in Patients with Clear Cell Renal Cell Carcinoma. J Nucl Med. 2024 Feb 22;65(5):740–3. https://doi: 10.2967/jnumed.123.267175
https://3b-pharma.com/

