In the field of radiopharmaceuticals, the process often begins with the identification of a biological target. This target is typically a specific protein found on the surface of a cell, known as the targeting protein. A corresponding moiety is then synthesized or biologically extracted to specifically bind to this protein – referred to as the targeting moiety. This moiety can be labelled either directly with a radioisotope, or indirectly. In this case, a linker can be used to put some distance between the radioactive site and the targeting moiety. This linker often enhances the physicochemical characteristics of the radiopharmaceutical. Each part of the “equation” is very important in choosing the right radioisotope and the radiolabeling approach that will follow. If this step is successful, then the idea is translated into action, so in vitro and in vivo experiments can follow.
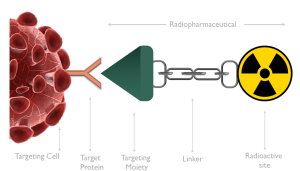
Figure 1: Basic structure of radiopharmaceuticals
There are different types of radioisotopes to choose from, depending on what the radiopharmaceutical is used for. Radioisotopes can be distinguished in several ways. We can categorize them according to their half-life into long-life and short-life isotopes (although in medicine we only use the short-life isotopes), according to their decay into PET, SPECT and therapeutic isotopes, or according to their nature, whether they are metallic or non-metallic isotopes.
Radiopharmaceutical chemists more often distinguish the isotopes by their nature, and we do so because we need to treat the non-metallic isotopes and the metallic isotopes differently. Their radiolabeling chemistry is significantly different from that of organic and inorganic chemistry. For example, most metallic isotopes are labelled by either metalation or transmetallation reactions (one-step synthesis), whereas non-metallic isotopes require more complex organic reactions to reach the final target (usually several steps).
There is a variety of metallic radioisotopes with similar coordination chemistry, so it is easier to switch from one metallic radioisotope to another. This is not possible with the non-metallic radioisotopes. Sometimes non-radioactive metals are even used to bind a non-metallic radioisotope to the same moiety that a metallic radioisotope normally binds.
Nevertheless, when non-metallic radioisotopes are used, the modification of the target moiety is usually minor or non-existent, whereas in most cases major modifications are required to incorporate a metal. For this reason, non-metallic isotopes are used more commonly used in the radiosynthesis of small molecules.
However, most of the radioisotopes used in clinical and pre-clinical research are not produced in-house. They therefore must follow a specific journey from their production site to their final destination. This journey rarely lasts less than a day, in most cases might take 1 to 3 days. Once it arrives, radiosynthesis follows soon enough to create the final structure of the radiopharmaceutical of interest.
Unfortunately, this journey can take even longer. The reasons for this are, firstly because the QC analysis of the radioisotope is not always performed the same day as production. There are cases where this procedure is carried out even 2 days later. If the QC is done just the day after, it’s delivery to the final destination will take up to 4 days instead of 3.
And the situation can get even worse because domestic flights are used to transport radioisotopes. And often (especially during summer), domestic flights band radioactive packages without any notice in advance. The cargo is then put on the next available flight! Or what if it arrives at the airport’s customs office at 15:30 or even later? Customs work daily from 08:00-16:00, so it is easy for the radioisotope to be stacked at the airport overnight or even over the weekend.
If we take all this into account and the radioisotope is 177Lu, if 100MBq of activity is produced on day 0, then we will get about 60MBq. And this is quite good for 177Lu, as this activity is still more than enough to perform experiments. The only issue is that you can use 177Lu for less time (~10 days instead of 2 weeks). But what happens if the isotope is 64Cu? A similar production would give us 0.14MBq, which is not enough for an in vivo study! So, we will have to request for an even higher production.

Figure 2: The radioisotope’s journey
And that is not the only issue that arises. There is also the question of invoicing. Does the company charge for the production activity? Does the company charge for the activity on the calibration date? Or does it charge for the activity on the arrival date? For many companies, the second and the later are the same. But for a lot of companies this is not the case, and the activity charged is far more than the activity received! And “charging” is not only about the cost. Also, the activity declared to the local nuclear commission to “enter” the laboratory is usually the activity found on the calibration date, which is an issue for many laboratories with small activity limits.
Finally, another (fortunately not very common) problem is production failure. This could mean that production never started or that the isotope did not pass all the QC tests. In such a situation, best case scenario, if the radioisotope is produced frequently, the experiment will be delayed for a few days. In cases where production of the isotope is less frequent, the experiment may need to be completely cancelled, and use of more animals will be required.
In conclusion, when trying to decide which supplier to get your isotope from, you need to consider what type of isotope it is and the maximum number of days that this isotope can be used. Then it is important to check the production date, whether the QC analysis is soon after production and the shipping details to get to your facilities, as well as how early you need to order the isotope. Different suppliers have different requirements, but to be flexible with clients and in case tumors grow slower or faster than expected, you need to have made the amendments for isotope presence in the lab.
A discussion must be held with the supplier, either the production company or a third party, about the plan in case of a failed production or delay in arrival. What will happen if the isotope does not arrive on time?
Finally, an important consideration is the form in which the radioisotope arrives. Is it in solution or solid? Is it carrier added or not? Will it require further purification before use? What is the size of s-vial, does it get to stuck in them? Considering all these parameters, you will need to calculate the dose required for an experiment. In addition, billing amendments need to be done taking into account the final amount of activity arriving at the laboratory and to the delivery note. The expected activity at arrival time needs to be stated on the delivery note to avoid “overcharging” of the facility with activity, and the CoA for its production should be in place and delivered to the installation or third party before the isotope arrives at the airport, as it is mandatory to clear customs.

