This study investigates the preclinical quantitative imaging performance of the β⁻-emitting radionuclide ¹¹¹Ag using the BIOEMTECH γ-eye™ High Throughput system. The aim was to establish a robust technical framework for accurate activity quantification and to support future theranostic development for this emerging isotope.
Collaboration: Study was conducted in collaboration with Mattia Asti and AUSL-IRCCS Reggio Emilia research team.
Why ¹¹¹Ag?
¹¹¹Ag combines therapeutic β⁻ emissions with three distinct gamma photopeaks (96.7, 245.4, 342.1 keV), making it a promising radionuclide for combined imaging and therapy. However, its multi-peak emission profile requires careful optimization to achieve SPECT quantitative performance.
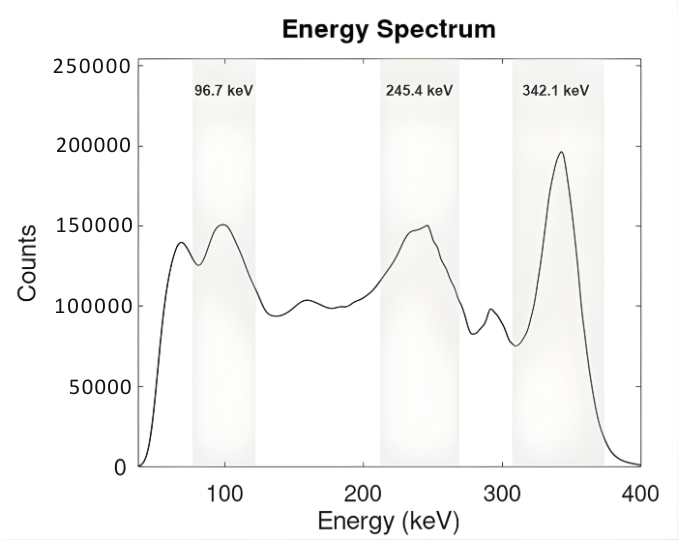 Figure 1: Averaged energy spectrum of ¹¹¹Ag
Figure 1: Averaged energy spectrum of ¹¹¹Ag
Extracted from the vial acquisitions. The three characteristic photopeaks at 96.7 keV, 245.4 keV and 342.1 keV are visible, confirming the expected γ-emission profile of ¹¹¹Ag. Acquisition time=60 minutes
Methodology
Using vial and mouse-phantom experiments, we performed a systematic optimization of the following parameters:
- energy-window selection across all photopeaks
- scatter-correction parameters using the Triple Energy Window (TEW) method, and
- energy-specific calibration for absolute activity quantification.
In addition, we evaluated multi-photopeak summation to increase sensitivity while maintaining quantitative accuracy.
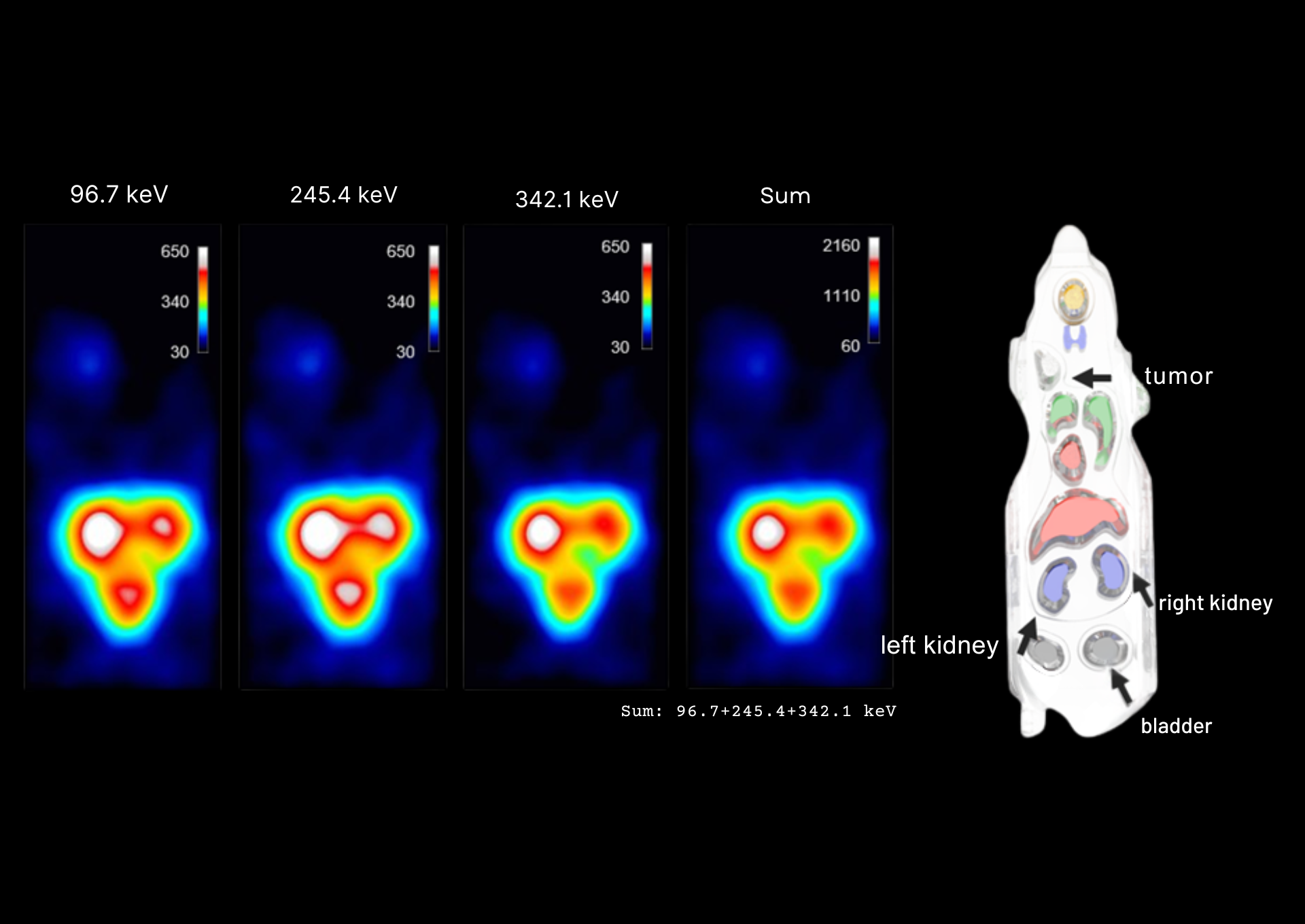
Figure 2: Mouse Phantom Imaging
Mouse phantom images produced at the three individual energy peaks (96.7 keV, 245.4 keV and 342.1 keV) and for the summed image of all three energy peaks. All images were generated using the optimal TEW scatter correction parameters (EW=±15%, W₁W₂=±10 keV). Acquisition time=60 minutes
Key results
The optimal configuration was defined as main energy windows of ±15% and side windows of ±10 keV for TEW. High linearity (R²>0.99) was observed across the full activity range, along with <10% quantification error in the mouse-phantom “organs.” Multi-photopeak summation provided ~2× sensitivity improvement without loss of accuracy, and enabled clear visualization of kidneys, urinary bladder and tumor regions in the mouse phantom.
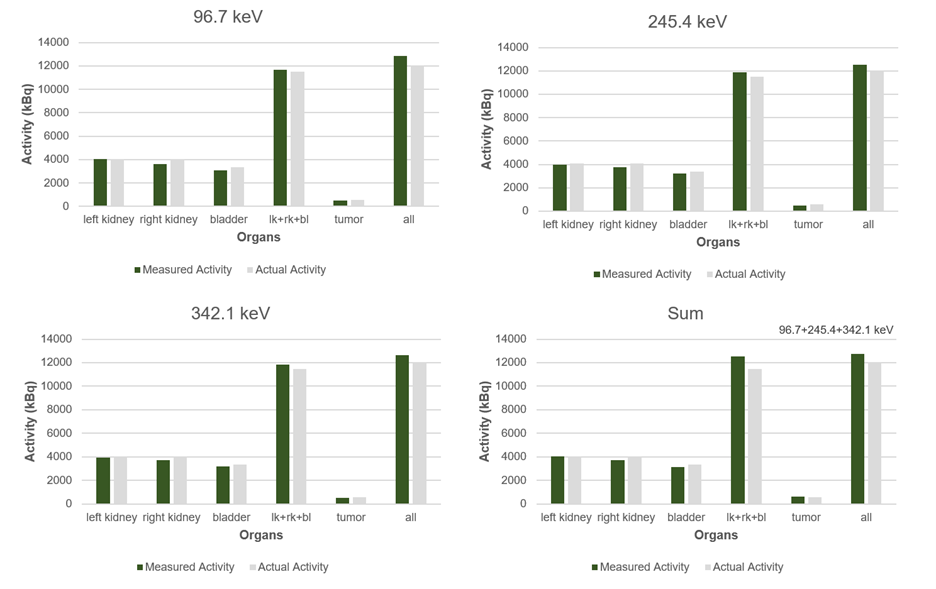 Figure 3: Measured and actual activity values (kBq)
Figure 3: Measured and actual activity values (kBq)
for the left kidney, right kidney, bladder, tumor, the combined left kidney, right kidney, bladder region (lk+rk+bl), and the full mouse phantom across the three energy windows (96.7, 245.4 and 342.1 keV) and for the summed image of all three energy peaks.
Significance and applications
By establishing practical acquisition and correction parameters, this work provides a quantitative foundation for reliable preclinical dosimetry, kinetic modelling of silver-based agents, cross-comparison with established theranostic radionuclides (e.g., ¹⁷⁷Lu, ¹¹¹In), and future translation of ¹¹¹Ag-based radiopharmaceuticals. A robust technical basis is essential prior to transitioning to in vivo studies.
