Highlights
- Simultaneous PET imaging of three healthy mice on the BIOEMTECH β-eye™ system
- Evaluation of image quality across different acquisition durations and post-injection time points
- Demonstration of a compact, multi-animal PET workflow
Introduction
Multi-animal PET imaging has the potential to significantly improve throughput in preclinical studies. However, simultaneous acquisition introduces technical challenges related to sensitivity, image quality and acquisition time, particularly when applied in compact, desktop imaging systems.
Here, we present an evaluation of simultaneous PET imaging of three mice using the BIOEMTECH β-eye™ system, focusing on qualitative image characteristics across different acquisition and post-injection conditions.
Study design
This study included three healthy mice imaged simultaneously in a single PET session. Imaging was performed under varying acquisition durations and post-injection time points following administration of ¹⁸F-FDG.
The objective was not to compare biological endpoints, but to explore the impact of acquisition parameters on image appearance in a simultaneous multi-animal configuration.
Imaging protocol
Each mouse received an intravenous injection of ¹⁸F-FDG with administered activity adjusted per acquisition condition. PET imaging was performed at different post-injection time points and scan durations, as outlined below:
- Condition 1: 6 min scan duration, 60 min post-injection, 2.5 MBq injected activity per mouse
- Condition 2: 12 min scan duration, 70 min post-injection, 2.34 MBq injected activity per mouse
- Condition 3: 18 min scan duration, 90 min post-injection, 2.06 MBq injected activity per mouse
Methods
Animals were positioned to enable simultaneous PET acquisition of three subjects within the system’s field of view. All scans were acquired using the BIOEMTECH β-eye™ preclinical PET system under identical reconstruction and visualisation settings to allow qualitative comparison across conditions.
Results
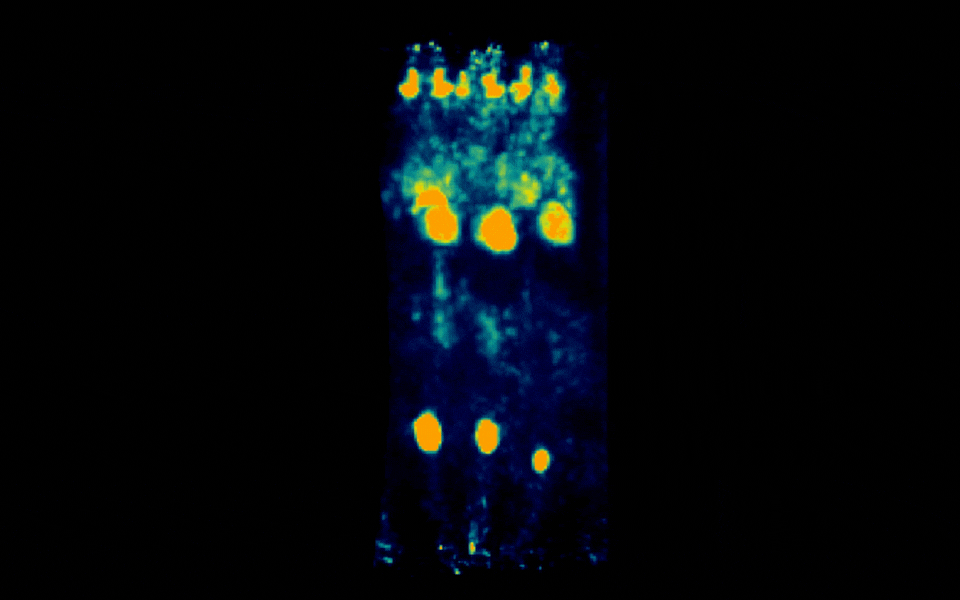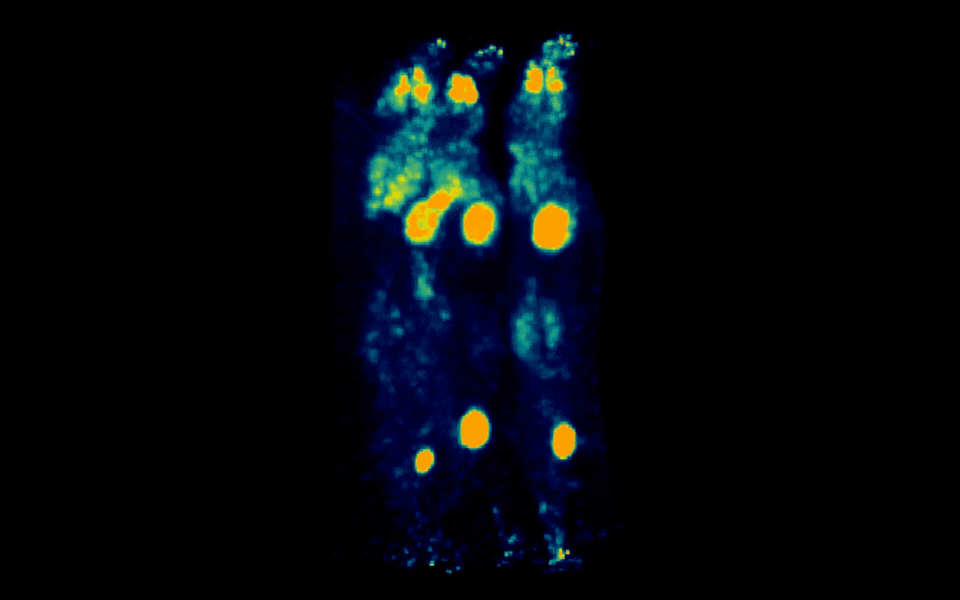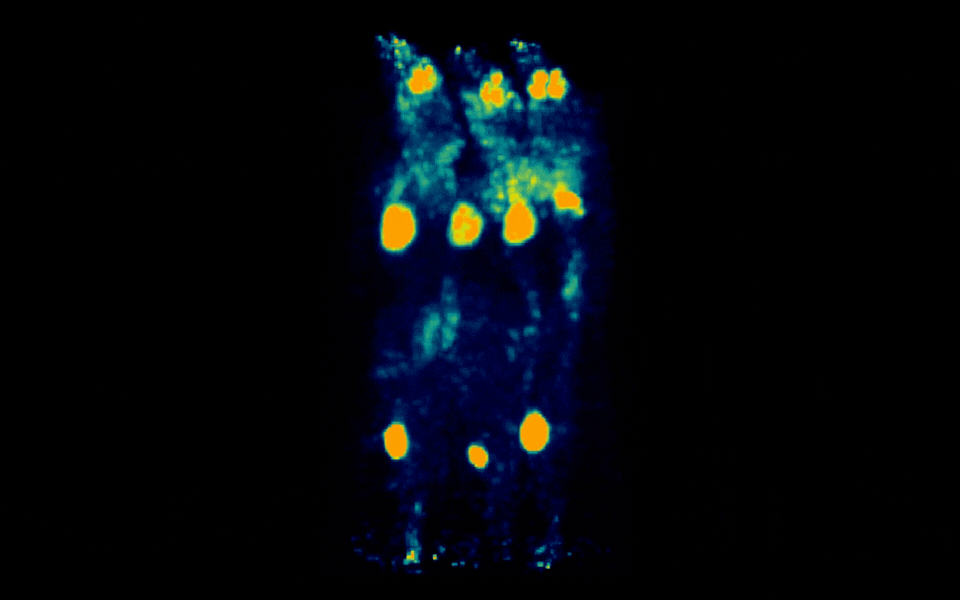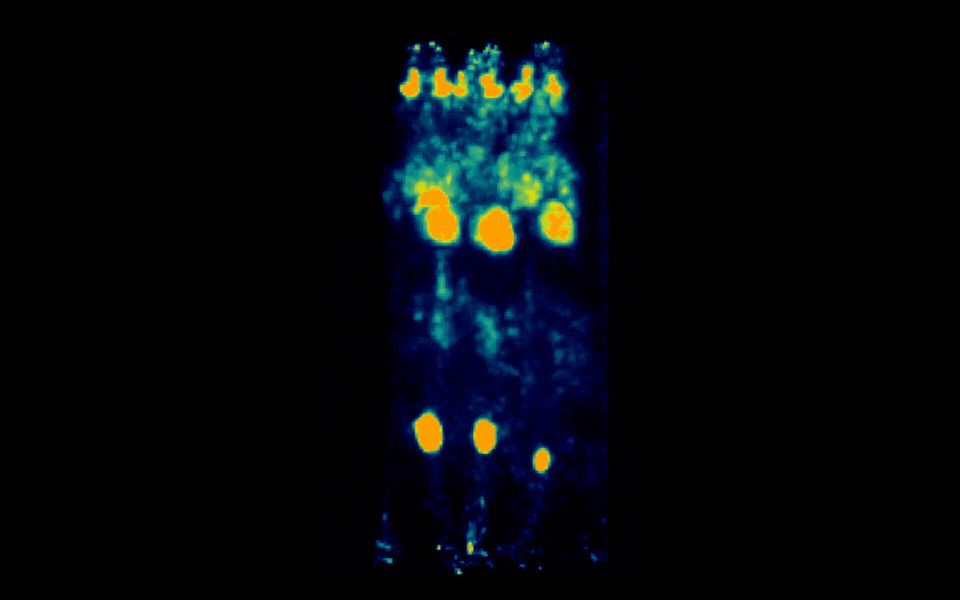
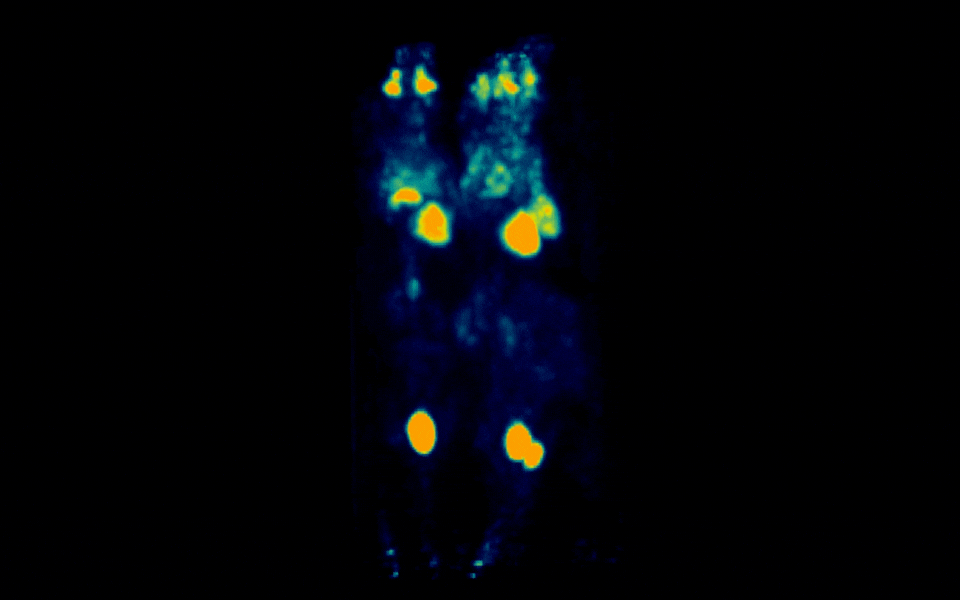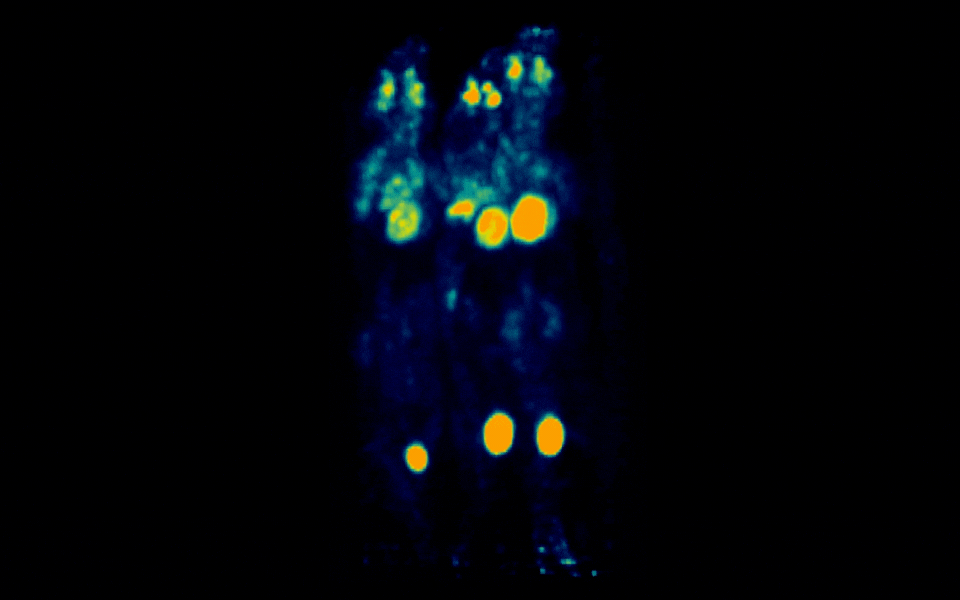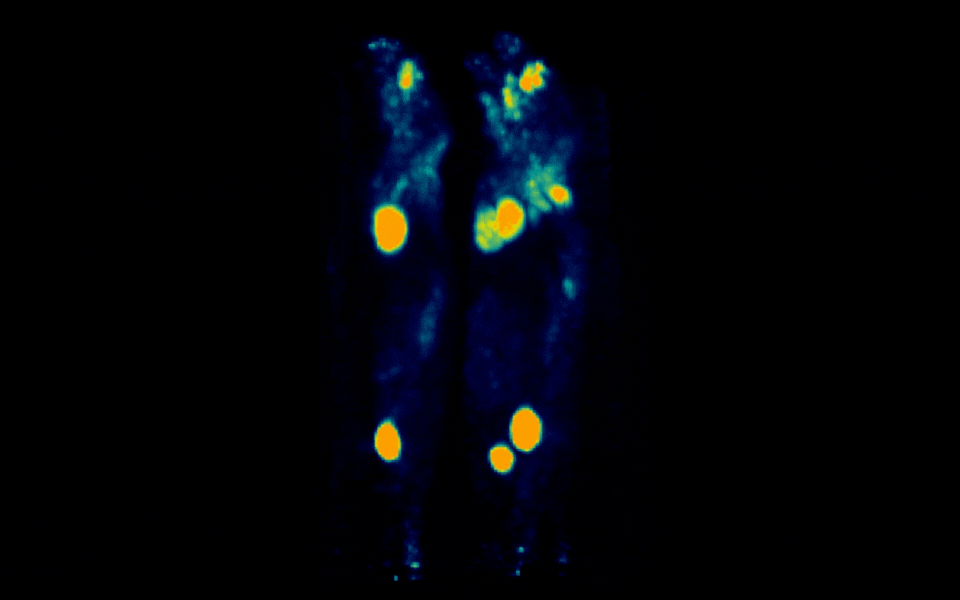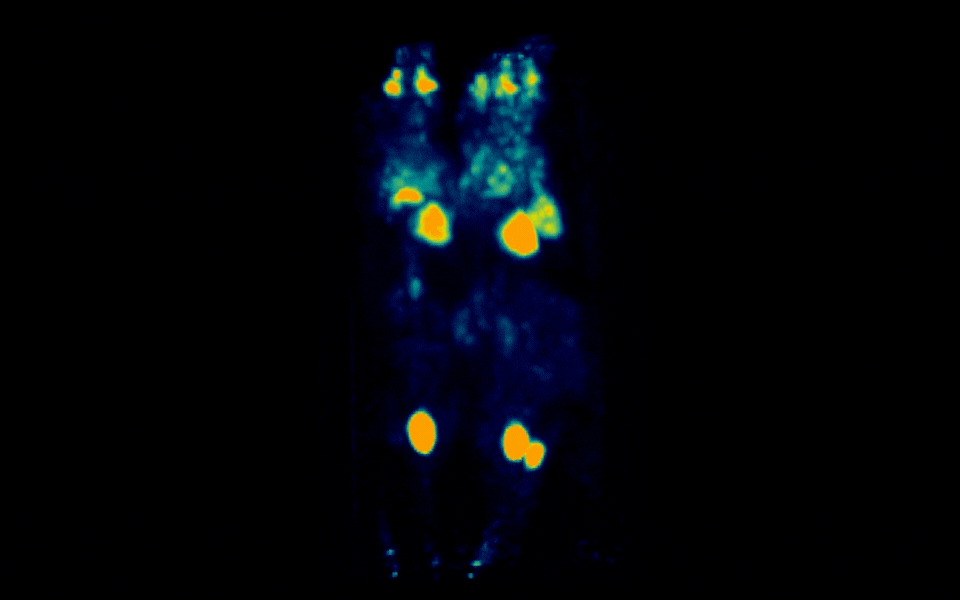
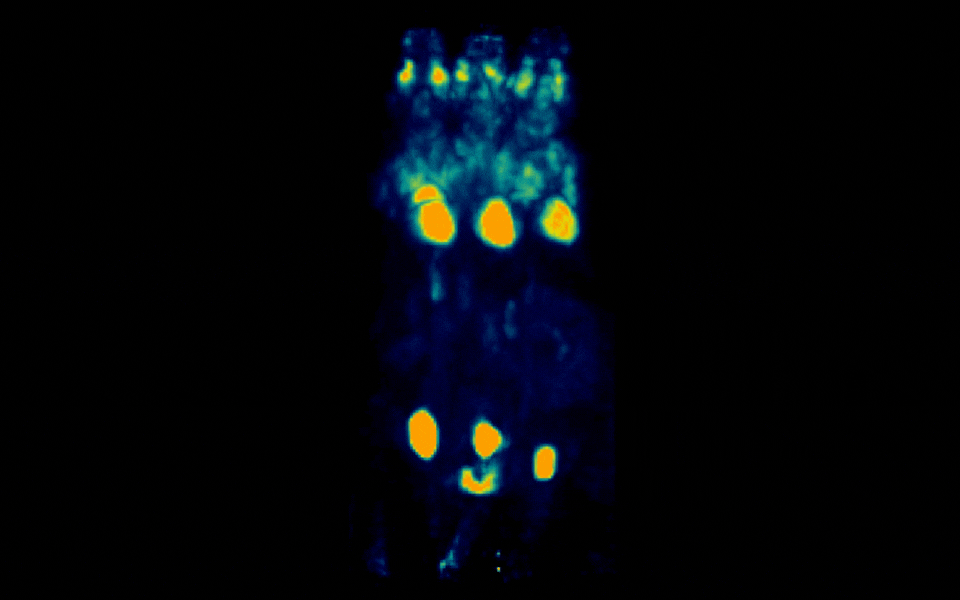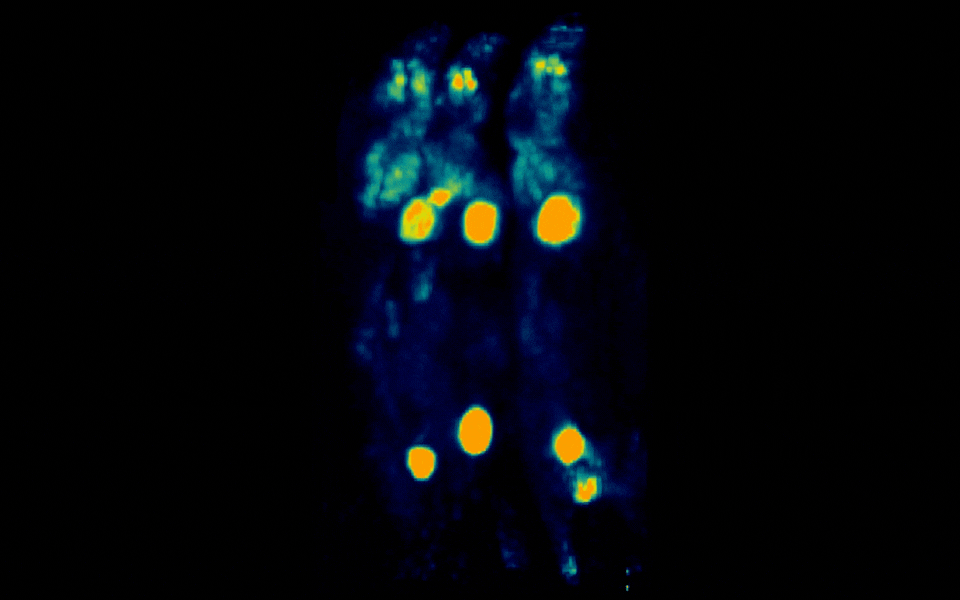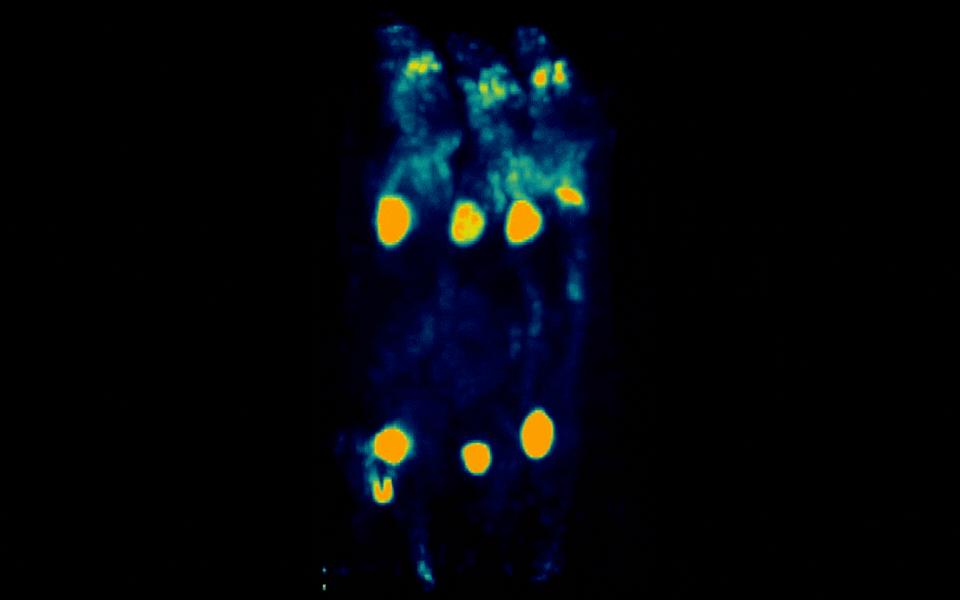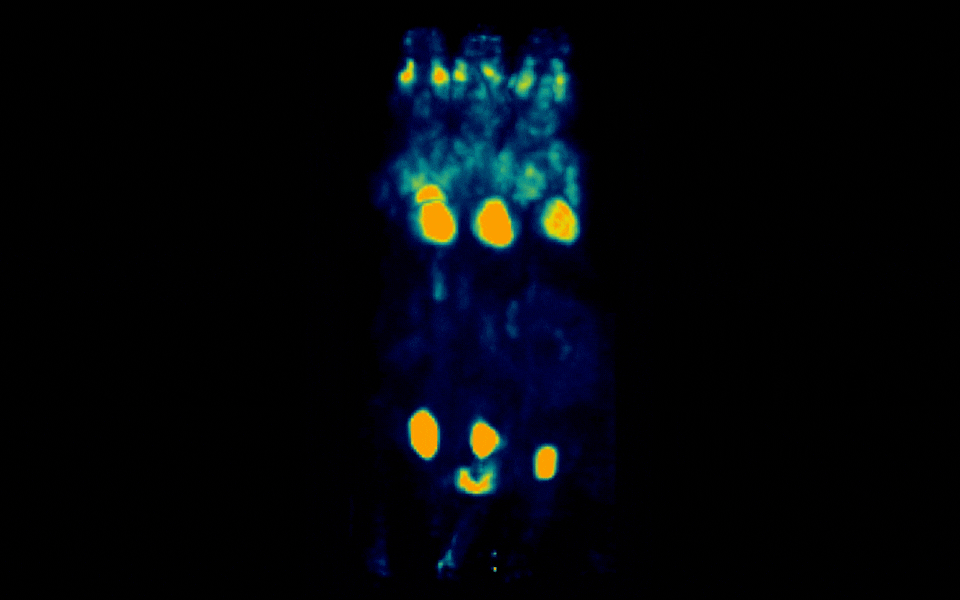
Representative images across all acquisition conditions showed the expected whole-body distribution of ¹⁸F-FDG, with visually prominent uptake in the myocardium (heart), as well as strong signal in the urinary bladder, consistent with renal clearance and also in salivary gland regions in the head.
Figure 1: Simultaneous PET imaging of three healthy mice acquired with a 6-minute scan duration, 60 minutes post-injection and an injected activity of 2.5 MBq per mouse.
Increasing scan duration and post-injection time resulted in visibly improved signal definition and reduced image noise, while shorter acquisitions demonstrated the feasibility of rapid multi-animal imaging with acceptable qualitative image quality.
Figure 2: Simultaneous PET imaging of three healthy mice acquired with a 12-minute scan duration, 70 minutes post-injection and an injected activity of 2.34 MBq per mouse.
Figure 3: Simultaneous PET imaging of three healthy mice acquired with a 18-minute scan duration, 90 minutes post-injection and an injected activity of 2.06 MBq per mouse.
Image processing and visual assessment
Image reconstruction and visualisation were performed using consistent parameters across all datasets. Qualitative assessment focused on overall signal distribution, contrast and noise characteristics, rather than quantitative endpoint extraction.
This approach allowed direct visual comparison of acquisition behaviour under different scan durations in a simultaneous three-animal configuration.
Conclusion
This early-stage evaluation demonstrates the technical feasibility of simultaneous PET imaging of three mice using a desktop preclinical PET system. Even under reduced scan durations and low injected activity per animal, diagnostically relevant whole-body ¹⁸F-FDG distribution could be visualised.
These findings support the potential of multi-animal PET acquisition as a throughput-enhancing strategy in preclinical research and provide a foundation for further optimisation and quantitative validation.