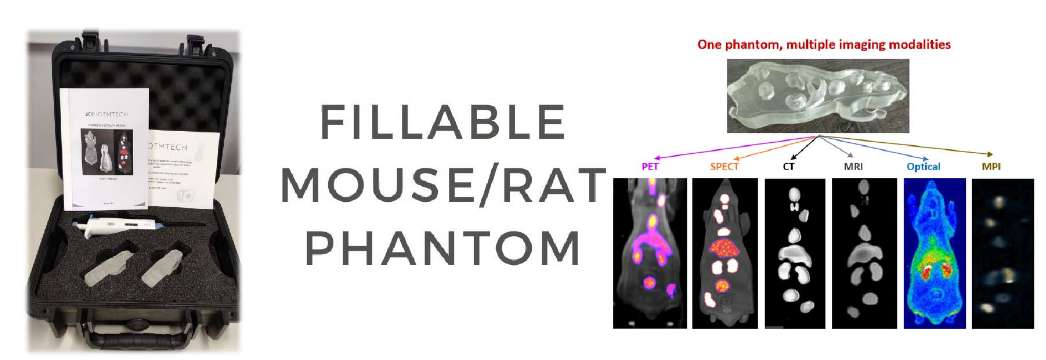
We are happy to see that our friends and collaborators at MOLECUBES NV used our fillable mouse to evaluate their new high sensitivity SPECT collimator. This work was presented in Society of Nuclear Medicine and Molecular Imaging (SNMMI) 2022 conference and the abstract is published at the Journal of Nuclear Medicine – JNM. We are glad to see that the fillable mouse is recognized as a reference phantom for the validation of imaging systems and processing algorithms!
28 August 2022
