According to Pearson’s Hard and Soft Acids and Bases (HSAB) theory, proposed in 1968, chemical species can be classified as either “hard” or “soft”, based on certain intrinsic properties. “Hard” species are typically small, have high oxidation states, and are weakly polarizable, while “soft” species tend to be larger, have lower oxidation states, and are highly polarizable. Simplifying this concept, chemical hardness can be quantitatively expressed as ½(I – A), where I is the ionization potential and A is the electron affinity of the system. Figure 1 presents a general classification of the elements in the periodic table according to this theory.
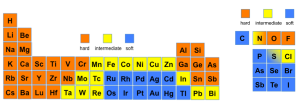
Figure 1: Classification of chemical element according to HSAB theory
It is worth noting that, while the majority of the commonly utilized radiometals are classified as “hard” according to the HSAB theory, several promising radionuclides currently under investigation for future theranostic applications, fall into the “soft” or “intermediate” categories. These radiometals include Silver-103/104/111 and Copper-61/64/67 triplets, as well as Mercury-197m/g and Lead-203/212 doublets. Figure 2 provides an overview of the primary decay modes and associated energies of these radionuclides.
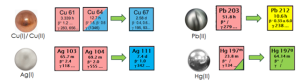
Figure 2: Main decay modes and energies of the soft/intermediate radionuclides mentioned in this paper.
In order to precisely deliver their cytotoxic radiation solely to tumor sites, radiometals have to be conveyed to their specific target by attachment to a molecular vector (small molecule, peptide, antibody …) that exhibits high affinity for that target. Typically, this connection is provided by a chelator, i.e. a chemical structure capable of tightly binding the radiometal and, in turn, being covalently linked to the biological vector. The HSAB theory can provide valuable insight into designing effective chelators for radiometals. According to the theory, “hard” metals tend to form strong interactions with “hard” donor atoms (such as oxygen and nitrogen), while “soft” metals preferentially form strong bonds with “soft” donor atoms (such as sulfur and phosphorus). As a result, widely known chelators (e.g., DOTA, NOTA, HBED, DFO*, DATA, THP, SAR, MACROPA, EDTA …) are highly effective at encapsulating commonly used radiometal ions like Ga-68, Y-90, Lu-177 and Zr-89, because both the radiometals and donor atoms in the chelators are classified as “hard.” However, these chelators often fail or prove suboptimal when coordinating with “soft” or “intermediate” radiometals, which require different chelation strategies to ensure stability and effectiveness. (Caution! This assumption is a simplification, as several other factors – such as geometry, metal charge, coordination number, ionic radius and the chelator’s structural framework – also play critical roles when evaluating ligand-metal interactions. All these parameters must be considered to accurately predict the effectiveness of a given chelator in forming stable complexes with radiometals).
This concept was emphasized by comparing the performance of DOTA – a highly versatile chelator – in complexing the aforementioned soft/intermediate radiometals, with that of modified DOTA derivatives, in which the carboxylate arms were partially or completely substituted with thioether groups to increase the softness of the structure (see Figure 3). The comparison focused on key metrics such as p[M] values, radiochemical incorporation and serum stability, providing insights into how these structural modifications influence chelation efficiency and complex stability.
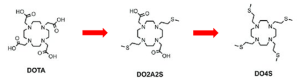
Figure 3: Structures of DOTA-derived softer chelators.
As a result, the performance of DOTA can be significantly enhanced by using DO2A2S and DO4S for intermediate radiometals (such as Pb-212 and Cu-64) and soft radiometals (such as Ag-111 and Hg-197m/g), respectively. For example, the labeling conditions – including temperature, incubation time and ligand concentration- as well as in serum stability demonstrated substantial improvements for DO2A2S with Cu-64 compared to DOTA. Meanwhile, DO4S showed high incorporation yield (>99%) with Ag-111 and Hg-197 where DOTA totally failed. Notably, a strong correlation was observed between the chemical softness of the radiometals and their corresponding chelators, further validating the utility of the HSAB theory in designing new chelating scaffolds.
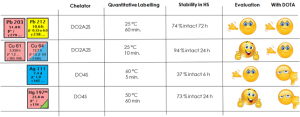
Further studies are mandatory for extending the stability of the Ag-111 and Hg-197m/g complexes in vivo. Nevertheless, the use of sulfur-rich tetra-azamacrocycle derivatives represents a pioneering example of chelators able to impressive performances in the coordination of soft radiometals.
References
Tosato, M.; Randhawa, P.; Asti, M.et al. Capturing Mercury-197m/g for Auger Electron Therapy and Cancer Theranostic with Sulfur-Containing Cyclen-Based Macrocycles, Inorg. Chem., 2024, 63, 30, 14241-14255
Tosato, M.; Randhawa, P.; Lazzari, L. et al. Tuning the Softness of the Pendant Arms and the Polyazamacrocyclic Backbone to Chelate the 203Pb/212Pb Theranostic Pair, Inorg. Chem., 2024, 63, 4, 1745-1758
Tosato, M.; Franchi, S.; Dalla Tiezza, M. et al. Tuning the Framework of Thioether-Functionalized Polyazamacrocycles: Searching for a Chelator for Theranostic Silver Radioisotopes, Inorg. Chem., 2023, 62, 20777-20790
Tosato, M.; Verona, M.; Favaretto, C. et al. Chelation of Theranostic Copper Radioisotopes with S-Rich Macrocycles: From Radiolabelling of Copper-64 to In Vivo Investigation, Molecules, 2022, 27, 13, 4158
Tosato, M.; Pelosato, M.; Franchi et al. When Ring Makes the Difference: Coordination Properties of Cu2+/Cu+ Complexes with Sulfur-Pendant Polyazamacrocycles for Radiopharmaceutical Applications, New J. Chem., 2022, 46, 10012-10025
Tosato, M.; Dalla Tiezza, M.; May et al. Copper Coordination Chemistry of Sulfur Pendant Cyclen Derivatives: An Attempt to Hinder the Reductive-Induced Demetallation in 64/67Cu Radiopharmaceuticals, Inorg. Chem., 2021, 60, 15, 11530-11547
Tosato, M.; Asti, M.; Dalla Tiezza, M. et al. Highly Stable Silver(I) Complexes with Cyclen-Based Ligands Bearing Sulphide Arms: A Step Towards Silver-111 Labeled Radiopharmaceuticals, Inorg. Chem., 2020, 59, 10907-10919

