The evolution of nuclear medicine over recent years has ushered in a transformative era for cancer therapeutics. By leveraging radioactive isotopes, it is now feasible to selectively target cancer cells with remarkable accuracy, offering promising strategies for treating cancers that are otherwise resistant to conventional therapies. Among the expanding repertoire of therapeutic radionuclides, alpha-emitting isotopes have garnered particular attention due to their highly localized cytotoxic effects and potent linear energy transfer.
Targeted alpha therapy (TAT) employs alpha-emitting isotopes coupled to (bio)molecules designed to recognize and bind cancer-specific biomarkers. This approach facilitates the delivery of genotoxic radiation doses at the cellular level while minimizing damage to surrounding healthy tissues. Despite its therapeutic potential, the clinical implementation of TAT has been constrained by practical limitations, namely: limited isotope availability, challenges in chelation chemistry, and the redistribution of radioactive daughter nuclides.
Among the most promising candidates to overcome these challenges is Lead-212 (Pb-212, t½ = 10.6 hours). While Pb-212 itself is a beta-emitter, its therapeutic relevance lies in its role as an in vivo generator of the alpha-emitter Bismuth-212 (Bi-212, t½ = 61 minutes) (Figure 1). The half-life of Pb-212 offers an optimal balance, being long enough to allow for radiolabeling, quality control, and patient administration, yet short enough to reduce prolonged radiation exposure.
From a chemical perspective, Pb-212 forms stable complexes with established chelators such as DOTA, TCMC, DOTAM, and PSC, facilitating efficient and reliable radiolabeling. Moreover, Pb-212 possesses intrinsic imaging capability via SPECT, attributable to X- and gamma ray emissions within its decay chain. This enables quantitative imaging of radioligand uptake in tumours and other organs, adding a functional imaging layer to therapeutic delivery.
An especially compelling feature of Pb-212 is its pairing with Lead-203 (Pb-203, t½= 51.9 hours) to form a true theranostic duo. Both isotopes are chemically identical and exhibit near identical in vivo pharmacokinetics, enabling accurate prediction of Pb-212-radioligand behavior through Pb-203-based SPECT imaging. This allows for non-invasive evaluation of tumour targeting, radiation dosimetry, and patient-specific treatment planning, all critical components in advancing personalized radiopharmaceutical therapy.
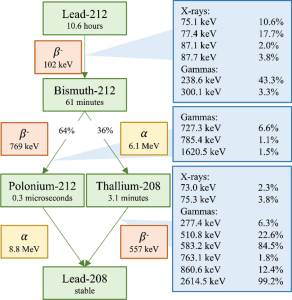
Figure 1: Decay scheme of Pb-212 outlining the alpha, beta and photon emissions
The production of Pb-212 is relatively reliable. It is routinely obtained from parent isotopes; Thorium-228 and Radium-224, via either emanation or acid elution generator systems. Both production systems are robust and scalable, laying the groundwork for broader clinical accessibility. At ARTBIO, we have developed a decentralized production model tailored to the properties of Pb-212. This model aims at bringing manufacturing closer to treatment sites to ensure timely delivery and maximize therapeutic window.
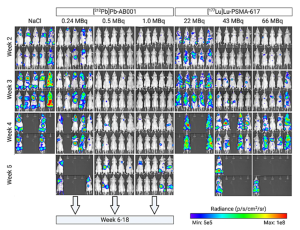
Preclinical and early clinical data continue to affirm the therapeutic promise of Pb-212. Studies have demonstrated significant anti-tumour efficacy in models of neuroendocrine tumours (NETs), small cell lung cancer (SCLC), and prostate cancer. One notable study has shown superior tumour growth control of Pb-212-AB001, a PSMA-targeting radioligand compared to the clinically validated beta-emitter Lu-177-PSMA-617 in a metastatic prostate cancer mouse model (Figure 2). Clinically, several early-phase trials are now underway, with initial findings suggesting strong efficacy in solid tumours and an encouraging safety profile, marked by manageable hematologic and renal toxicities.
As nuclear medicine continues to evolve, therapeutic isotopes such as Pb-212 are poised to play a central role in the next generation of oncologic care. With its favorable half-life, versatile radiochemistry, established production routes, and growing clinical evidence base, Pb-212 stands as a keystone isotope in the advancement of targeted alpha therapy.
Figure 2: Bioluminescence representation of metastatic tumour burden over time in mice treated with different doses of 212Pb-AB001 and 177Lu-PSMA-617, when compared to untreated controls.
References
Kvassheim, M. et al. Quantitative SPECT/CT imaging of lead-212: a phantom study. EJNMMI Phys 9, 52 (2022). https://doi.org/10.1186/s40658-022-00481-z
Christensen K.A et al. Targeting CXCR4 with 212Pb/203Pb-Pentixather Significantly Increases Overall Survival in Small Cell Lung Cancer. Radiat Res. 2025 Jul 3. doi: 10.1667/RADE-24-00232.1. Epub ahead of print. PMID: 40605199
Høyvik, A.J.K. et al. Therapeutic evaluation of [212Pb]Pb-AB001 and [177Lu]Lu-PSMA-617 in a mouse model of disseminated prostate cancer. Eur J Nucl Med Mol Imaging (2025). https://doi.org/10.1007/s00259-025-07330-y
Berner K et al. First-in-Human Phase 0 Study of AB001, a Prostate-Specific Membrane Antigen-Targeted 212Pb Radioligand, in Patients with Metastatic Castration-Resistant Prostate Cancer. J Nucl Med. 2025 May 1;66(5):732-738. doi: 10.2967/jnumed.124.269299. PMID: 40081958; PMCID: PMC12051763
Michler E. et al. [203/212Pb]Pb-VMT-α-NET as a novel theranostic agent for targeted alpha radiotherapy—first clinical experience. Eur J Nucl Med Mol Imaging (2025). https://doi.org/10.1007/s00259-025-07269-0
Delpassand E.S et al. Targeted α-Emitter Therapy with 212Pb-DOTAMTATE for the Treatment of Metastatic SSTR-Expressing Neuroendocrine Tumors: First-in-Humans Dose-Escalation Clinical Trial. J Nucl Med. 2022 Sep;63(9):1326-1333. doi: 10.2967/jnumed.121.263230. Epub 2022 Jan 6. PMID: 34992153; PMCID: PMC9454455