Actinium-225 is one of the most promising alpha-emitters for targeted alpha therapy. This is mostly due the relatively useful emission characteristics (Figure 1), but also the future prospects for upscalability of production. Whilst the current amount of Actinium-225 is limited, it is predicted that by 2032 the production capacity could be enough to treat more than 500.000 patients per year [1]. Currently more than 27 molecules that incorporate Actinium-225 is registered in clinical trials [2].
There is a possibility of 5 production routes for Actinium-225, and when radiolabelling is attempted, it is important to understand the influence of the route of production on the method employed. Different routes have different radionuclidic impurities for which has to be tested, furthermore the routes might have long lived contaminants and there might be differences in specific activity or contamination with non-radioactive metals [2,3]. All of this will influence the efficiency of the chelator to capture Αctinium-225, dictating possible changes in radiolabelling methods. This will also have an influence on the quality control methods that needs to be tested for radionuclidic and radioisotopic impurities. Currently, most published protocols use Actinium-225 produced from Thorium-229 generators which yields a very pure Actinium-225 in high specific activity. However, other routes will become more available and more information on the use of these sources will result in the future.
When Actinium-225 production is started out, it is important that safety of the operator should be the number one priority. Internal exposure to Actinium-225 (through ingestion) is the major concern, hence it is important to control the contamination of surfaces, the operator and the environment. The external radiation by daughter decay is of less concern, and thin lead shielding should be sufficient to protect against these risks. Critically, during the performance of QC, it is also important to ensure that these activities do not lead to the uncontrolled release of Actinium-225 in the air or on surfaces that could lead to ingestion. Additionally, it is difficult to measure contamination with alpha-emitters, so it is important to obtain specialised instruments that can also measure alpha radiation. For the performance of QC such as radiochemical purity, where the daughter radionuclides Bismuth-213 and Francium-221 is used, it is important that the strength of these signals is relatively low.
Actinium-225 are incorporated in small molecules, peptides and larger biological vectors through chelation. DOTA is the standard chelator currently being used in the clinic, but recently phase 1 trials have been launched using MACROPA as well. MACROPA affords milder labelling conditions (temperature and incubation time) which could be more suitable for larger biological vectors [4]. As with other radiometal based radiopharmaceuticals, it is important that metal contamination in precursors and raw materials is kept as low as possible, with the method also not introducing additional metals as this could lead to poor labelling yields. As a final precaution, DTPA is added post-labelling to clear up all unlabeled daughter alpha-emitters and Actinium-225 should it be present. DTPA promotes clearance of these through the renal system [5].
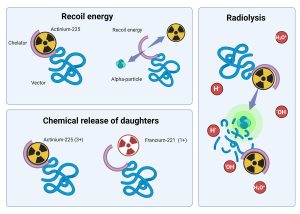
Figure 1: Possible mechanisms for instability of Αctinium-225 radiopharmaceuticals (drawn with liscenced version of Biorender.com)
The quality control procedures of Actinium-225 radiopharmaceuticals is being complicated by the lack of direct measurement of alpha-emitters through these procedures [6]. As such, the daughter radionuclides are measured as surrogates for Actinium-225. Before attempts are being made to implement Actinium-225 production, it is important to consider the standard practices as is outlined in the following guideline: “production and quality control of Actinium-225 radiopharmaceuticals” [2]. Since Actinium-225 radiopharmaceuticals can have multiple mechanisms of instability (recoil, different coordination properties of daughter radionuclides, radiolysis – Figure 1) it is critical to validate all production methods and also the stability of radiopharmaceutical formulations not only through ITLC, but also radioHPLC. For stabilization it is important to add quenchers and radioprotectors to the final formulation, and sometimes even during the radiolabelling procedure. Methods have been published and this is also in area of stability of 225Ac-radiopharmaceuticals and optimal analytical methods, with attempts at direct measurements of Actinium-225 also being underway [5,7].
Actinium-225 is a very promising alpha-emitter, with already strong advancement in the implementation in the Nuclear Medicine Clinic. Once availability increases, we can be sure to see many more innovative theranostic molecules bringing hope to patients with radioresistant disease.
References
[1] Zimmermann R. 2023. Is actinium really happening? J Nucl. Med. 64(10): 1516-1518. DOI: 10.2967/jnumed.123.265907.
[2] IAEA. Production and quality control of actinium-225 radiopharmaceuticals. DOI: 10.31092/iaea.95h3-2ji2.
[3] Radchenko A et al. 2021. Production and supply of α-particle-emitting radionuclides for targeted α-therapy. J Nucl med, 62(11): 1495-1503. DOI: 10.2967/jnumed.120.261016.
[4] Thiele NA et al., 2017. An eighteen-membered macrocyclic ligand for actinium-225 targeted alpha therapy. Angew Chem Int Ed Engl. 56(46): 14712-1471. DOI: 10.1002/anie.201709532.
[5] Hooijman et al., 2024. Implementing Ac-225 labelled radiopharmaceuticals: practical considerations and (pre-)clinical perspectives. EJNMMI Radiopharmacy and Chemistry 9:9. DOI: 10.1186/s41181-024-00239-1.
[6] Kleynhans J & Duatti A. 2022. The determination of the radiochemical purity of actinium-225 radiopharmaceuticals: a conundrum. EJNMMI Radiopharmacy and Chemistry, 7:23. DOI: 10.1186/s41181-022-00175-y.
[7] Hooijman et al., 2021. Development of [225Ac]Ac-PSMA-I&T for targeted alpha therapy according to GMP guidelines for treatment of mCRPC. Pharmaceutics, 13:715. DOI: 10.3390/pharmaceutics13050715.

